Are you considering the AGGA dental device but feeling overwhelmed by conflicting information? You’re not alone. In 2025, a staggering 65% of patients report confusion about jaw expansion treatments. Don’t worry – we’ve got you covered.
This guide tackles the top questions about AGGA dental devices, providing expert answers to help you make an informed decision. We’ll explore:
- What AGGA claims to do and how it works
- Potential benefits and risks backed by recent studies
- The latest legal and FDA developments
- Expert opinions from leading orthodontists
- Alternative treatments you should know about
Whether you’re just starting your research or seeking a second opinion, you’ll find clear, up-to-date information here. Ready to unravel the AGGA mystery and take control of your dental health? Let’s dive in.
What is the AGGA Dental Device?
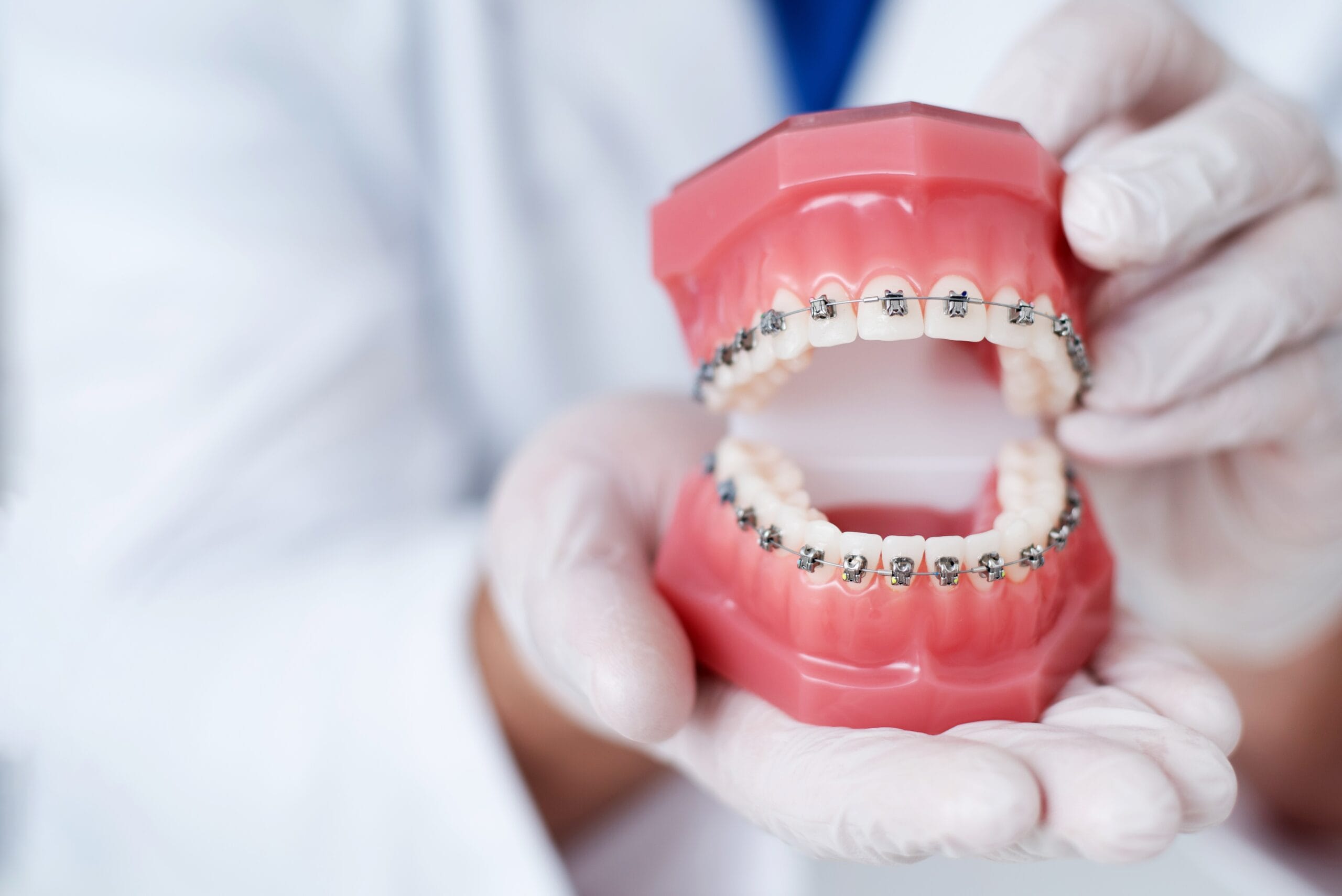
The AGGA is a dental appliance designed to promote forward growth of the upper and lower jaws in adult patients. It consists of molar bands, springs, and a wire framework that applies pressure to the palate and upper front teeth. The device’s inventor, Dr. Steve Galella, claims it can remodel adult jaws without surgery, potentially addressing issues like sleep apnea, TMJ disorders, and facial aesthetics.
How Does the AGGA Device Work?
According to proponents, the AGGA works by:
- Applying gentle, consistent pressure to the palate
- Stimulating the nasopalatine nerve
- Encouraging forward growth of the maxilla (upper jaw)
- Creating space for the mandible (lower jaw) to advance
The device is typically worn for several months, with regular adjustments by a trained dentist.
Common Questions About AGGA
Is the AGGA FDA-approved?
No, the AGGA is not FDA-approved. In fact, recent investigations have revealed that the device was never submitted for FDA review. This lack of regulatory oversight has raised significant concerns about its safety and efficacy.
What conditions is AGGA claimed to treat?
Proponents suggest AGGA can address:
- Sleep apnea
- TMJ disorders
- Facial asymmetry
- Breathing issues
- Orthodontic problems
However, it’s important to note that these claims are not supported by peer-reviewed scientific evidence.
How much does AGGA treatment cost?
AGGA treatment typically costs between $7,000 to $10,000. However, given the ongoing controversies and potential risks, many dental insurance plans do not cover this treatment.
How long does AGGA treatment take?
Treatment duration varies, but patients often wear the device for 12-18 months. Regular adjustments are required throughout this period.
Expert Opinions on AGGA
Dental specialists and orthodontic experts have expressed serious concerns about the AGGA device:
Dr. Marianna Evans, orthodontist and periodontist: “The concept behind AGGA contradicts our understanding of adult jaw anatomy. Once growth is complete, you cannot significantly alter jaw position without surgery.”
Dr. Michael Stosich, orthodontist: “There’s no scientific evidence supporting the claims made about AGGA. Adult jaw expansion without surgery is simply not possible.”
Potential Risks and Complications
Patients and dental professionals have reported several serious complications associated with AGGA use:
- Tooth movement and flaring
- Gum recession
- Root exposure
- Bone erosion
- Tooth loss
- Chronic pain
- Bite misalignment
These issues can lead to permanent damage requiring extensive and costly corrective treatments.
Legal and Regulatory Concerns
The AGGA device has become the subject of numerous lawsuits and regulatory scrutiny:
FDA Investigation
The U.S. Food and Drug Administration (FDA) has announced it is “evaluating safety concerns” related to the AGGA and similar devices. The agency has requested that patients and healthcare providers report any complications experienced with these appliances.
Ongoing Lawsuits
At least 20 patients have filed lawsuits against AGGA’s inventor, manufacturer, and the Las Vegas Institute, which trained dentists in its use. These legal actions allege that the device caused severe dental damage and facial disfigurement.
Alternative Treatment Options
For patients considering jaw realignment or addressing sleep apnea, TMJ, or orthodontic issues, several evidence-based alternatives exist:
- Orthodontic treatment (braces or clear aligners)
- Orthognathic surgery
- CPAP therapy for sleep apnea
- Custom-fitted oral appliances for TMJ disorders
It’s crucial to consult with a board-certified orthodontist or maxillofacial surgeon to discuss appropriate treatment options based on your individual needs.
What Patients Should Know
If you’re considering AGGA treatment or have already undergone the procedure, keep these points in mind:
- Seek multiple opinions: Consult with several dental specialists, including orthodontists and oral surgeons, before committing to any treatment.
- Ask for evidence: Request peer-reviewed studies supporting the safety and efficacy of any proposed treatment.
- Understand the risks: Ensure you’re fully informed about potential complications and long-term consequences.
- Check credentials: Verify the qualifications and experience of any practitioner recommending or performing AGGA treatment.
- Consider alternatives: Explore evidence-based treatments that have a proven track record of safety and effectiveness.
- Report complications: If you’ve experienced issues with an AGGA device, report them to the FDA through their MedWatch program.
The Future of AGGA and Similar Devices
As investigations continue and lawsuits progress, the future of AGGA and similar devices remains uncertain. The dental community eagerly awaits:
- Results of the FDA’s safety evaluation
- Outcomes of ongoing legal proceedings
- Potential new regulations for dental devices
These developments will likely shape the future use and availability of AGGA and related appliances.
Conclusion: Prioritizing Patient Safety and Evidence-Based Dentistry
The controversy surrounding the AGGA dental device underscores the importance of evidence-based practices in dentistry. While the allure of non-surgical jaw expansion is understandable, the potential risks and lack of scientific support raise serious concerns.
Patients should approach any novel dental treatment with caution, especially those lacking FDA approval and peer-reviewed evidence. Always prioritize your oral health and overall well-being by choosing treatments with a proven track record of safety and efficacy.
As the dental community continues to evaluate the AGGA device, staying informed and consulting with qualified specialists remains the best approach for patients seeking jaw alignment or related treatments.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with a qualified dental professional before undergoing any dental treatment or procedure.
The Anterior Growth Guidance Appliance (AGGA) is a dental device created to assist with breathing issues, TMJ, crossbites, and other conditions related to jaw alignment. It applies light pressure to stimulate jaw growth or “remodeling”. However, its effectiveness and safety have been subjects of debate and legal scrutiny.
The AGGA is designed for forward jaw expansion, while the DNA appliance focuses on lateral growth. The DNA appliance is also removable and does not require retainers after treatment, unlike the AGGA, which is a fixed appliance.
Yes, there have been safety concerns with the AGGA, including reports of chronic pain, tooth dislocation, and bone erosion. The U.S. Food and Drug Administration (FDA) is currently evaluating the safety of this and similar dental devices.
Before choosing AGGA treatment, consider its fixed nature, the lengthy treatment duration, its relatively unproven track record, and ongoing FDA investigations. It’s crucial to consult with a trusted dental professional to understand all potential risks and alternatives.
Alternatives to the AGGA include the DNA appliance, ALF, and Homeoblock devices. These appliances have different mechanisms of action and are often preferred due to their proven track records and focus on holistic jaw growth.
The AGGA is typically recommended for adults and adolescents whose jaws have finished growing. Using the device in young children whose bones are still developing may carry additional risks and is generally not advised without close supervision by a qualified provider.
Most patients will need to wear a retainer following AGGA treatment to maintain the new jaw position and prevent relapse. Retainers may be removable or fixed, and may need to be worn for several years or indefinitely.
Brushing and flossing around the AGGA appliance can be challenging. Patients may need to use special tools like interdental brushes or water flossers to maintain good oral hygiene and prevent cavities or gum disease.
There have been reports of tooth loss, root resorption, and other dental complications associated with the AGGA. The risk of damage may be higher in cases of improper use or pre-existing dental issues.
Many patients report discomfort or pain, especially in the initial stages of treatment as the jaws begin to shift. Over-the-counter pain medication and soft foods can help manage symptoms.
AGGA treatment typically takes 6-12 months or longer, depending on the individual case. However, some patients may need to wear the device for several years to achieve desired results.